![]()
Nitrogen gas surrounds us all, yet we barely notice this benign compound exists (78% of the atmosphere is composed of nitrogen gas, N2). However, when combined with other elements, nitrogen can have a very schizophrenic personality. Nitrogen compounds can be used to feed, or kill, with equal ease. This ironic nature of nitrogen was tragically demonstrated in the Oklahoma City Bombing when fertilizer was used as an explosive, killing hundreds.
"Enough already...go to the bottom."
All living things require nitrogen to live (it is the "amino" in "amino acids", a major component in DNA, RNA, and proteins) however few creatures can make direct use of the sea of nitrogen surrounding us all. This is because the two nitrogen atoms that make up a nitrogen molecule are held firmly together by a triple bond which is exceedingly difficult to break.
Because of this bond, nitrogen gas simply does not participate in any reactions at room temperature (or even at the higher temperatures found in small fires), and is therefore described as inert. Only at extremely high temperatures (such as those found near a lightning bolt or in an automobile's engine), or through the magic of "nitrogen fixing" bacteria (who work their trick with a complex set of enzymes instead of heat), can this triple bond energy be overcome, making nitrogen gas momentarily reactive while in an excited state.
If this excited nitrogen molecule is combined with oxygen (which incidentally composes the rest of the atmosphere) nitrogen oxide (NO) is produced. This readily oxidizes to nitrogen dioxide (NO2), which provides the brownish haze seen in smog (as all Los Angeles residents are well aware).
If, on the other hand, the excited nitrogen gas combines with hydrogen, it forms ammonia (NH3). And ammonia, unlike smog, is a very useful compound indeed. It can be used to make fertilizers, high explosives, nitric acid, and household cleaning agents.
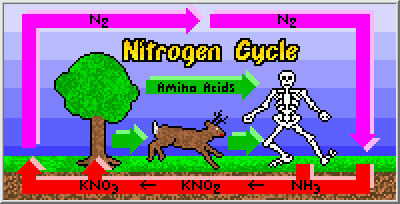
Converting nitrogen gas into more reactive (and useful) nitrogen compounds, such as ammonia, encompasses the first stage in the "nitrogen cycle". Once converted from its gaseous form, fixed nitrogen compounds allow plants to grow large and healthy. Animals gain access to this nitrogen by eating the plants, and deposit excess nitrogen in their feces. Fixed nitrogen is also returned to the soil when plants and animals die. Bacteria then decompose this organic matter first into ammonia, then into nitrites (like potassium nitrite: KNO2), and finally into nitrates (like potassium nitrate: KNO3), which are again used by plants. Additional bacteria return some of the fixed nitrogen back to the atmosphere (in the form of nitrogen gas), thereby regulating the whole cycle.
For thousands of years, humans had little impact on the nitrogen cycle. The strong bond found in nitrogen gas prevented its simple conversion to other, much more useful, nitrogen compounds. People were therefore entirely dependent upon bacteria for the initial nitrogen fixation. Once fixed in the cycle, nitrogen compounds could be collected.
One of the best, and largest, sources of this fixed nitrogen was found in Chile. This outcrop was due to a vast number of sea birds which nested, and went to the bathroom, along its coasts. Over thousands of years these "natural" deposits called "Guano" accumulated and became several feet thick. A huge industry developed to supply this Chilean saltpeter to the rest of the world.
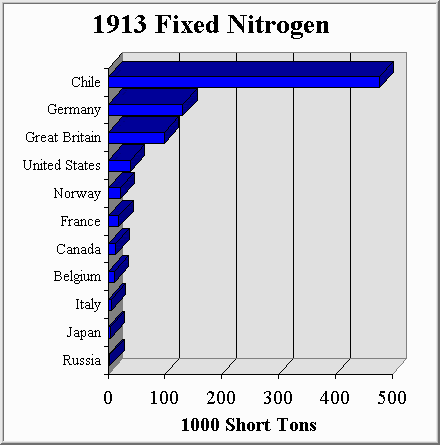
Figure 4-1, Source: (M3)
With synthetic production almost non-existent, the world was entirely dependent on the Chilean resource for fertilizers and high explosives. This was a fact which military leaders did not overlook. They realized that if war broke out, the countries which lacked (or were denied) access to the Chilean supply (like Germany) would quickly run out of munitions.
In 1913 if you were an up and coming nation, intent on feeding your people, or conquering your enemies through conquest, you needed as much Chilean saltpeter as possible. In short, the fate of the world depended upon who could get their hands on the most bird shit. It is therefor no coincidence that the first major naval battle of World War I occured off the coast of Chile.
Shortly before the outbreak of World War I, two patriotic Germans developed a method for producing synthetic ammonia. The first plants using this "Haber-Bosch Process" were constructed shortly after the outbreak of the war. They had discovered that ammonia could be made by placing nitrogen gas and hydrogen gas in a high pressure chamber. With the addition of a suitable catalyst, and a little heat to speed things up, vast quantities of fixed nitrogen could be produced. Without the Haber-Bosch Process, Germany would have run out of munitions in 1916 thereby ending the war.
To compete with this, other countries copied the process and quickly scaled up their own synthetic ammonia production capabilities. When the war was over, fixed nitrogen continued to be produced in large amounts because of its use as a fertilizer. By 1934 Chile was supplying only 7% of the worlds fixed nitrogen (a huge drop from the 56% supplied in 1913). Synthetic ammonia production had arrived in force.
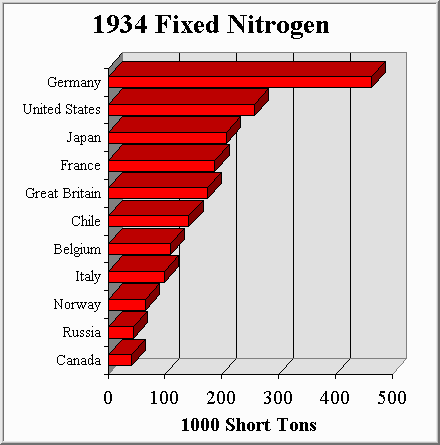
Figure 4-2, Source: (M3)
Tabulated data for previous two graphs is shown below...
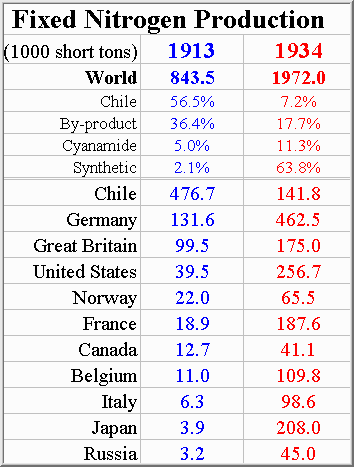
Figure 4-3, Source: (M3)
As these figures show, synthetic ammonia production eliminated the worlds dependence upon Chilean saltpeter. Chemical engineers played a large role in designing, building, and operating the ammonia plants that made this possible. In 1934 if you wanted to feed your country or wage a war you turned to a chemical engineer. This fact brought with it a moral dilemma for the chemical engineer asked to build an ammonia plant...
![]() Amino
Acids are the building blocks of life. All living things are
composed of only 20 such compounds, with the amino group (NH2)
common to them all.
Amino
Acids are the building blocks of life. All living things are
composed of only 20 such compounds, with the amino group (NH2)
common to them all.
![]() Nitrous
Oxide (dinitrogen oxide) (N2O) Otherwise known as laughing
gas, this compound is a colorless gas at room temperatures and pressures.
Nitrous
Oxide (dinitrogen oxide) (N2O) Otherwise known as laughing
gas, this compound is a colorless gas at room temperatures and pressures.
![]() Nitrogen
Oxide (NO) A colorless bi-product formed in internal combustion engines
where high temperatures and pressures are capable of combining the nitrogen
and oxygen gases found in the air.
Nitrogen
Oxide (NO) A colorless bi-product formed in internal combustion engines
where high temperatures and pressures are capable of combining the nitrogen
and oxygen gases found in the air.
![]() Nitrogen
Dioxide (NO2) Otherwise known as smog, this brown
gas comes about as nitrogen oxide is spontaneously oxidized in the atmosphere.
Nitrogen
Dioxide (NO2) Otherwise known as smog, this brown
gas comes about as nitrogen oxide is spontaneously oxidized in the atmosphere.
![]() Potassium
Nitrate (KNO3) Often referred to as saltpeter, it
can be placed directly on the soil as a fertilizer, or when mixed
with sulfur and coal forms gunpowder. Saltpeter is prized by
both farmers and generals.
Potassium
Nitrate (KNO3) Often referred to as saltpeter, it
can be placed directly on the soil as a fertilizer, or when mixed
with sulfur and coal forms gunpowder. Saltpeter is prized by
both farmers and generals.
![]() Trinitrotoluene
(TNT, CH3C6H2(NO)3) A high
explosive used as the explosive charge in shells and bombs.
Trinitrotoluene
(TNT, CH3C6H2(NO)3) A high
explosive used as the explosive charge in shells and bombs.
![]() Nitroglycerin
(C3H5(ONO2)3) Being the principle
explosive ingredient in dynamite, this chemical packs quite a punch.
It is three times as powerful as an equal amount of gunpowder, is smokeless,
and its explosive wave travels 25 times faster. Because of its many uses,
and high demand, dynamite producers were able to amass great fortunes.
Alfred Nobel was one early manufacturer, and his fortune continues
to finance the Nobel Prize Award today.
Nitroglycerin
(C3H5(ONO2)3) Being the principle
explosive ingredient in dynamite, this chemical packs quite a punch.
It is three times as powerful as an equal amount of gunpowder, is smokeless,
and its explosive wave travels 25 times faster. Because of its many uses,
and high demand, dynamite producers were able to amass great fortunes.
Alfred Nobel was one early manufacturer, and his fortune continues
to finance the Nobel Prize Award today.
"The end already...go back to the top."
We always welcome COMMENTS, SUGGESTIONS, OR REACTIONS.