![]()
Petroleum refineries are marvels of modern engineering. Within them a maze of pipes, distillation columns, and chemical reactors turn crude oil into valuable products. Large refineries cost billions of dollars, employ several thousand workers, operate around the clock, and occupy the same area as several hundred football stadiums. The U.S. has about 300 refineries that can process anywhere between 40 and 400,000 barrels of oil a day. These refineries turn out the gasoline and chemical feedstocks that keep the country running.
"Enough already...go to the end."
Locating an oil field is the first obstacle to be overcome. The first explorers used Y-shaped devining rods and other supernatural, but ineffective, means of locating petroleum. Today geologists and petroleum engineers employ more tried and true methods. Instruments to aid the search include; geophones (uses sound), gravimeters (uses gravity), and magnetometers (uses the Earth's magnet field). While these methods narrow the search tremendously, a person still has to drill a exploratory well, or wildcat well, to see if the oil actually exists. Success brings visions of gushers soaring skyward, however today wells are capped before this happens.
There are three main types of drilling operations; cable-tool, rotary, and off-shore. Cable-tool drilling involves a jack-hammer approach were a chisel dislodges earth and hauls up the loose sediment. Rotary drilling works at much greater depths, and involve sinking a drill pipe with a rotating steel bit in the middle. Off-shore drilling involves huge semisubmersible platforms which lower a shaft to the ocean floor, containing any oil which is located.
All crude oil contains some amount of methane or other gases dissolved in it. Once the drilling shaft makes contact with the oil it releases the pressure in the underground reservoir. Just like opening a can of soda pop, the dissolved gases fizz out of solution pushing crude oil to the surface. The dissolved gases will allow about 20% recovery of oil. To get better recovery water is often pumped into the well, this forces the lighter oil to the surface. Water flooding allows recoveries of about 50%. The addition of surfactant allows even more oil to be recovered by preventing much of it from getting trapped in nooks and crannies. Yet, it is impossible to get all of the oil out of a well.
Because crude oil is a liquid it is much easier to move than natural gas or coal. Coal is nice and dense, so it does not require large holding containers, but it cannot be pumped. Conveyor belts and cranes cannot compete with pipelines for economic efficiency. Natural gas can be pumped using expensive compressors, but it requires enormous holding tanks. A recent trick has been to inject huge amounts of water into salt strata. The water dissolves the salt, leaving truly enormous caverns. The natural gas is then pumped in and stored until needed. The ease in transporting oil is one of the reasons we have become so dependent upon it. Pound for pound natural gas and coal just cannot compete.
The proven reserves of crude oil within the U.S. are about 3.9 billion cubic meters. This could cover the state of Minnesota with a layer one half inch thick. A reasonable value for the total amount of crude oil obtainable using current methods from around the world is 350 billion cubic meters. This could cover Minnesota with a layer of oil four and a half feet thick. Yet, at the rate we are consuming oil, the nation's reserves will be depleted by 2010, and the world's reserves will be depleted by the end of the 21st Century.
Yet, oil is not the only source of hydrocarbons. Natural gas and coal are both available in much greater amounts (see Distillation Figure). However, we may decide that it is not such a good idea to burn all of these hydrocarbons. Carbon dioxide is a strong greenhouse gas (along with water and methane), and the results to the global environment could be catastrophic to human life. Nuclear fission, solar power, hydroelectric power, and geothermal power offer immediate alternatives, however energy produced by these methods would be more expensive than burning oil, coal, or natural gas. The holy grail of power production, nuclear fusion, continues to elude scientists and engineers. In any case, refining techniques will remain vital to produce not only fuels but raw materials for petrochemical industries (plastics, pharmaceuticals, agrochemicals, etc.).
Quote by Ernest Bevin at the British Parliament during a heated discussion concerning the Middle East.
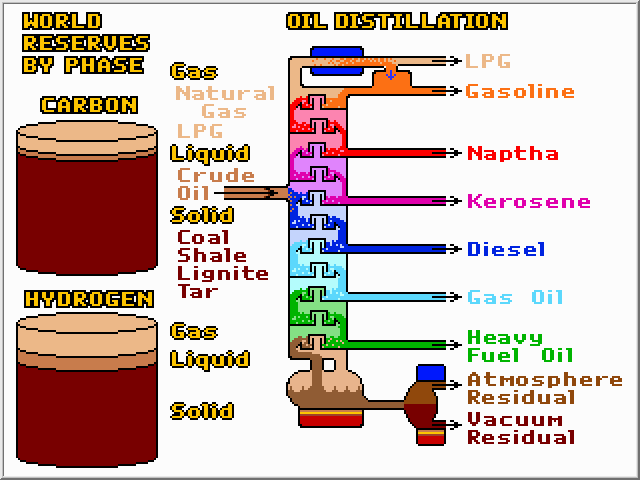
Oil contains a complex mixture of hydrocarbons. The first step in obtaining something of value from this muck is to de-salt and de-water it. Then the oil is heated and sent into a huge distillation column operating at atmospheric pressure. Heat is added at the reboiler, and removed at the condenser, thereby separating the oil into fractions based upon boiling point. A typical atmospheric column can separate about 4,000 cubic meters (25,000 barrels) of oil per day. The bottom fraction is sent to another column operating at a pressure of about 75 mm Hg (one tenth of an atmosphere). This column can separate the heaviest fraction without thermally degrading (cracking) it. Whereas atmospheric columns are thin at tall, vacuum columns are thick and short, to minimize pressure fluctuations along the column. Vacuum columns can be over 40 feet in diameter!
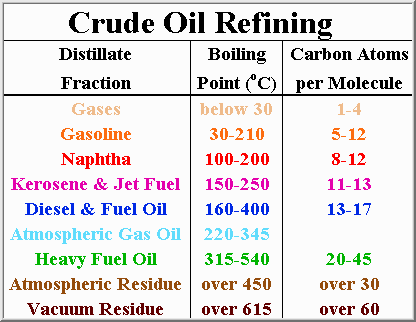
Various fractions are more important at different times of year. During the summer driving months, the public consumes vast amounts of gasoline, whereas during the winter more fuel oil is consumed. These demands also vary depending upon whether you live in the frigid north, or the humid south. Modern refineries are able to alter the ratios of the different fractions to meet demand, and maximize profit.
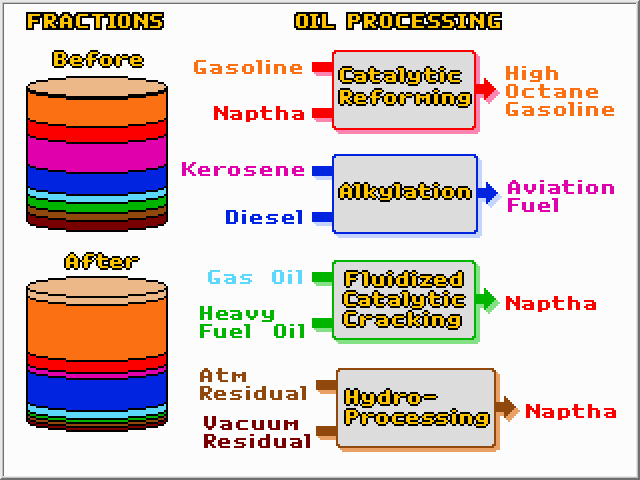
While distillation can separate oil into fractions, chemical reactors are required to create more of the products that are in high demand. Refineries rely on four major processing steps to alter the ratios of the different fractions. They are; Catalytic Reforming, Alkylation, Catalytic Cracking, and Hydroprocessing. Each of these methods involves feeding reactants to a reactor where they will be partly converted into products. The unreacted reactants are then separated from the products with a distillation column. The unreacted reactants are recycled for another pass, while the products are further separated and mixed with existing streams. In this way complete conversion of reactants can be obtained, even though not all of the reactants are converted on a given pass through the reactor. The four processing methods, along with distillation, are the pillars of petroleum refining.
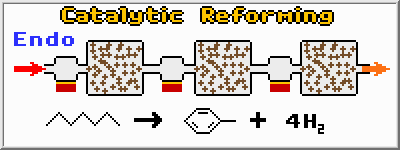
Catalytic Reforming produces high octane gasoline for today’s automobiles. Gasoline and naphtha feedstocks are heated to 500 degrees Celsius and flow through a series of fixed-bed catalytic reactors. Because the reactions which produce higher octane compounds (aliphatic in this case) are endothermic (absorb heat) additional heaters are installed between reactors to keep the reactants at the proper temperature. The catalyst is a platinum (Pt) metal on an alumina (Al2O3) base. While catalysts are never consumed in chemical reactions, they can be fouled, making them less effective over time. The series of reactors used in Catalytic Reforming are therefore designed to be disconnected, and swiveled out of place, so the catalyst can be regenerated.
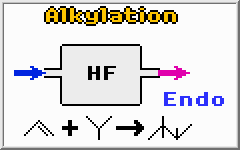
Alkylation is another process for producing high octane gasoline. The reaction requires an acid catalyst (sulfuric acid, H2SO4 or hydrofluoric acid, HF) at low temperatures (1-40 degrees Celsius) and low pressures (1-10 atmospheres). The acid composition is usually kept at about 50% making the mixture very corrosive.
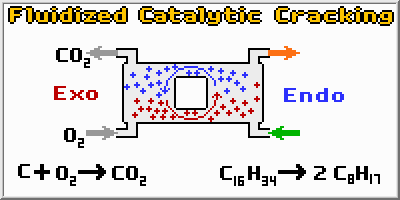
Catalytic Cracking takes long molecules and breaks them into much smaller molecules. The cracking reaction is very endothermic, and requires a large amount of heat. Another problem is that reaction quickly fouls the Silica (SiO2) and alumina (Al2O3) catalyst by forming coke on its surface. However, by using a fluidized bed to slowly carry the catalyst upwards, and then sending it to a regenerator where the coke can be burned off, the catalyst is continuously regenerated. This system has the additional benefit of using the large amounts of heat liberated in the exothermic regeneration reaction to heat the cracking reactor. The FCC system is a brilliant reaction scheme, which turns two negatives (heating and fouling) into a positive, thereby making the process extremely economical.
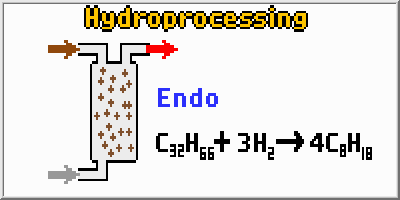
Hydroprocessing includes both hydrocracking and hydrotreating
techniques. Hydrotreating involves the addition of hydrogen atoms to molecules
without actually breaking the molecule into smaller pieces. Hydrotreating
involves temperatures of about 325 degrees Celsius and pressures
of about 50 atmospheres. Many catalysts will work, including; nickel,
palladium, platinum, cobalt, and iron. Hydrocracking breaks longer molecules
into smaller ones. Hydrocracking involves temperatures over 350
degrees Celsius and pressures up to 200 atmospheres. In both
cases, very long residence times (about an hour) are required because
of the slow nature of the reactions.
![]()
"The end already...go back to the top."
We always welcome COMMENTS,
SUGGESTIONS, OR REACTIONS.