![]()
The chemical engineer is often faced with complex mixtures of chemicals. In these mixtures some chemicals are valuable while others may be worthless or even hazardous. The trick is to separate the good from the bad without spending too much money along the way. While there are quite a few separation techniques in a chemical engineer's bag of tricks, distillation is the workhorse of the chemical industry. It is fairly inexpensive and can produce very high purity products. Because of this the petroleum industry has adopted it as their separation method of choice. The towers pointing skyward at oil refineries are in fact distillation columns, and their vast numbers reveal just how frequently this unit operation is used.
"Enough already...go to the end."
![]()
But how does a distillation column work? To answer this we will first observe how the world behaves, then try to understand why it works the way it does. Finally we must figure out how we can use this knowledge for our benefit. Following are descriptions of two experiments which will hopefully illuminate the physical principles governing distillation.
Imagine filling a pot full of water (1 kg or 2.2 lbs) and placing it on the stove. We turn on the burner (power of about 5 kW) and start heating the water, hoping to eventually bring it to a boil. While it is still cold lets stick a thermometer into the water so we may watch how the temperature changes during the process. Here is what happens (ignore the math if you like):
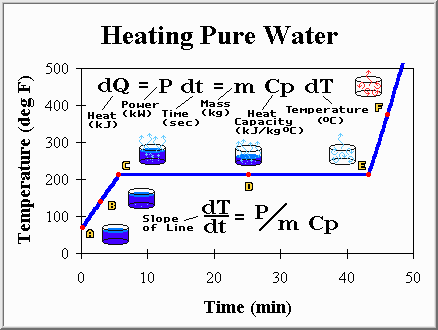
Point A: The water has just been placed on the burner. It is at the temperature of the tap (70 degrees Fahrenheit), but with the addition of heat from the burner it will not stay there long. Because the temperature is below the boiling point the liquid is called "sub-cooled".
Point B: The water is slowly warming up. The water obviously has a capacity to absorb heat and displays a temperature increase while absorbing that heat (heat capacity of 4.2 kJ/kg C).
Point C: The first bubble (of water vapor) appears at the bottom and rises to the surface. The bubble rises because steam is less dense than water. This is to say that a given volume of vapor is always lighter than the same volume of liquid. Gravity assures that the heavier fluid will displace the lighter fluid, and a good thing or filling a drinking glass with water would be a challenging process indeed.
Point D: More and more of the water is boiling off, being converted from water to steam. No surprise there, however something unusual has happened to our thermometer. It seems to have stopped rising, and hovers at 212 degrees Fahrenheit (100 degrees Celsius). The steam boiling off is also at 212 degrees Fahrenheit. Yet, the burner is still on, and is still much hotter than the water, so heat is still flowing into the water. It seems as though when a compound transforms from a liquid to a vapor some additional heat is absorbed. This heat does not raise the temperature, instead it causes some water to change to steam. Joseph Black observed this behavior in 1765 and called it "hidden heat". Today it is called "latent heat" but the idea is the same. Some heat, in fact a very large amount as evident by the long time needed to finish boiling, is required to turn water into steam (2257 kJ/kg). Similarly, steam gives off the same amount of heat when it is converted back to water. But enough talk, lets continue to watch the pot and see what happens as we add more and more heat.
Point E: The last drop of water boils away leaving us a pot full of steam and air. The temperature now begins to increase once again and the steam becomes "super-heated". The temperature grows rapidly because steam has a lower heat capacity than water and most of the vapor has left the pot so there is less material to heat up. Most cooks would remove the pot to prevent damaging it, but let's leave it on the burner to see what happens.
Point F: The temperature of the vapor within the pot continues to rise. It will increase until the pot, and vapor within it, finally reach the same temperature as the burner. At this point, no more heat will flow and the temperature will remain at a steady state.
![]() Pure
compounds have a capacity to absorb heat, and in the process
warm up.
Pure
compounds have a capacity to absorb heat, and in the process
warm up.
![]() Pure
compounds boil when they reach a temperature called their boiling
point. The temperature then remains constant, even though heat
is still being added, until all the liquid is boiled away.
Pure
compounds boil when they reach a temperature called their boiling
point. The temperature then remains constant, even though heat
is still being added, until all the liquid is boiled away.
![]() While
boiling, heat is absorbed, but no temperature increase is observed in the
liquid or the vapor. This hidden heat is called latent heat.
While
boiling, heat is absorbed, but no temperature increase is observed in the
liquid or the vapor. This hidden heat is called latent heat.
![]() Once
all the liquid is boiled off the temperature of the steam
will again increase, until the heat source and the steam are at
the same temperature.
Once
all the liquid is boiled off the temperature of the steam
will again increase, until the heat source and the steam are at
the same temperature.
Well, that was mildly amusing. We are now standing in a hot humid room and have a warped pot laying upon the burner. But, on the bright side, we understand the universe a little better and are one step closer to setting up a distillation column. Now, lets put a mixture of liquids in a pot and repeat the same experiment. We choose a bottle of whiskey, and pour it into the pot. The whiskey is made of half ethanol (ethyl alcohol) and half water. However, it is not entirely clear what is going to happen when we heat the mixture, because pure ethanol boils at 173 degrees Fahrenheit (78.3 degrees Celsius), not 212 degrees like pure water. Will the temperature remain constant while the mixture boils off? With this question in mind we eagerly turn on the burner and watch the thermometer. Our findings are summarized below:
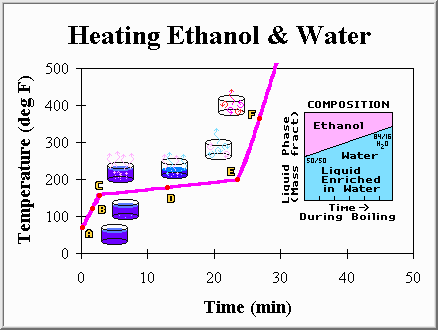
Point A: The mixture of ethanol and water has just been placed on the burner. The liquid is still cool, and for a moment we consider stopping the experiment to take a sip.
Point B: The mixture is warming up faster than the pure water did. This is not too surprising as we know that pure ethanol would warm up much faster than pure water. Ethanol's heat capacity (2.8 kJ/kg C) is smaller than that of water, and we expect the ethanol-water mixture to have properties somewhere between that of the pure components.
Point C: The first bubble appears at the bottom and rises to the surface. If we could catch this bubble we would find that it is enriched in ethanol. While the liquid is 50% ethanol and 50% water, the first bubble of vapor is over 65% ethanol. This may come as a surprise, but makes some sense... Because ethanol has a lower boiling point it has a tendency to boil off first. This temperature (about 176 degrees Fahrenheit) is called the bubble point, because it is the temperature at which the first bubble forms.
Point D: Ethanol, and water, continues to be boiled off. However, the temperature is not remaining constant. Instead, it has slowly been increasing. The latent heat is still present, and is responsible for slowing the temperature rise, but its presence is not nearly as obvious as when we had only pure water. The temperature is rising because the liquid phase is being enriched in water, which has a higher boiling point. This liquid enrichment occurs because the first vapors were mainly ethanol, and so a larger fraction of water was left behind.
Point E: The last drop of liquid is very rich in water, and it too eventually boils away. This is called the dew point because if we were condensing the vapor instead of boiling the liquid this would be the temperature at which the first drop of liquid would form (about 185 degrees Fahrenheit). That first drop of liquid condensed would be mostly (84%) water.
Point F: The temperature of the vapors within the pot continues to rise until they are as hot as the burner.
![]() Mixtures
have a capacity to absorb heat, and in the process become warmer.
Mixtures
have a capacity to absorb heat, and in the process become warmer.
![]() Mixtures
boil when they reach a temperature called their bubble point.
Afterwards the temperature slowly rises, even though latent heat is still
present, until the last drop of liquid vaporizes at the dew point.
Mixtures
boil when they reach a temperature called their bubble point.
Afterwards the temperature slowly rises, even though latent heat is still
present, until the last drop of liquid vaporizes at the dew point.
![]() The
vapor produced at the bubble point is rich in the lower
boiling compound (in this case ethanol).
The
vapor produced at the bubble point is rich in the lower
boiling compound (in this case ethanol).
![]() The
last little bit of liquid is rich in the higher boiling
compound (in this case water).
The
last little bit of liquid is rich in the higher boiling
compound (in this case water).
![]() Once
all the liquid is boiled off the temperature of the vapor mixture
will again increase.
Once
all the liquid is boiled off the temperature of the vapor mixture
will again increase.
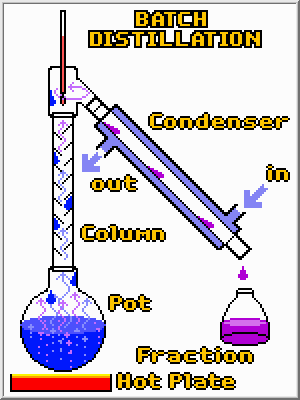
It is fairly easy to turn pots and burners into a batch distillation apparatus. A condenser is required to turn the vapors back to a liquid so they can be easily collected. A tall column is also desirable because it greatly improves the separation by giving high boiling compounds another chance to condense before they reached the top and are collected. Finally, by using different collection vessels, the original mixture can be separated into fractions. However, despite these bells and whistles the principle is the same; by applying heat a distillation column separates compounds in a mixture based upon their boiling points.
One of the characteristics of the Industrial Revolution has been a shift from small scale batch (craft like) operations to large scale continuous (plant based) mass production. Ford's automotive assembly line is the typical example of mass production, but the same kinds of changes also occurred in the chemical industry. Labor intensive batch distillation was replaced with continuous distillation which allowed a much greater chemical throughput. Just as mass production techniques greatly reduced the price of a "Model T" automobile, it also greatly reduced the price of the gasoline which powered that machine.
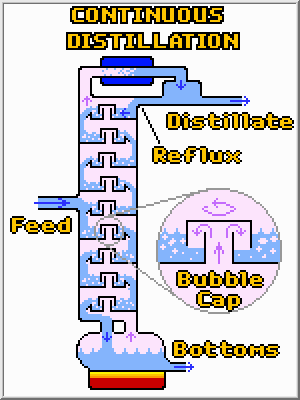
There are two major types of continuous distillation columns, but both operate in basically the same way. In both cases liquid is continuously fed into the column, and at least two streams (distillate and bottoms), together containing the same amount of total material, are continuously removed. Heat is added to the re-boiler (pot) and removed at the condenser. The re-boiler vaporizes some of the liquid, which then follows a treacherous path to the top of the column where it is re-condensed. Along the way most of the high boiling compounds will been left behind, and the distillate will be quite pure. To further aid the separation process some of the liquid distillate is often returned to the column where it flows back to the bottom. Along the way this reflux condenses some of the higher boiling liquids out of the vapor phase helping to purify the vapor. The two types of columns are:
![]() Tray
Columns (shown above): Such columns consist of physically separated
pools of liquid which are in intimate contact with a vapor. Bubble columns
are often used to force the upward flowing vapor through these pools of
downward flowing liquid. Each of these trays operates as an equilibrium
stage (like the pot and water examples above).
Tray
Columns (shown above): Such columns consist of physically separated
pools of liquid which are in intimate contact with a vapor. Bubble columns
are often used to force the upward flowing vapor through these pools of
downward flowing liquid. Each of these trays operates as an equilibrium
stage (like the pot and water examples above).
![]() Packed
Columns: Such columns are filled with a saddle shaped packing that
resembles Styrofoam peanuts. This packing provides a lot of surface area
for the vapor to condense upon and assures that the liquid and vapor are
in intimate contact.
Packed
Columns: Such columns are filled with a saddle shaped packing that
resembles Styrofoam peanuts. This packing provides a lot of surface area
for the vapor to condense upon and assures that the liquid and vapor are
in intimate contact.
Whereas the composition of the distillate and bottoms in batch distillation changes over time, a continuous column operates under steady conditions where the composition at a given location does not change over time. This steady state operation is desired in almost all continuous unit operations. Because the composition only depends upon the position in the column, additional product steams can be easily tapped at different heights (not shown) and each tray will have a different composition of compounds. The trays at the top of the column are rich in light boiling compounds while those at the bottom are rich in compounds that only boil at high temperatures.
"The end already...go back to the top."
We always welcome COMMENTS,
SUGGESTIONS, OR REACTIONS.